Abstract
Introduction
Clinical trials are essential in ensuring that new treatments are safe and effective and are also a way to improve standard of care. Patients enrolled in clinical trials receive optimal clinical care due to the close management included in trial design. Using the 2020 Lymphoma Coalition (LC) Global Patient Survey (GPS) on Lymphomas and CLL, this study aims to provide insight to the experience of patients with lymphoma and CLL who have participated in a clinical trial compared to those who have not. This study will focus on 1) their experience and disease management 2) their involvement in healthcare decision-making, and 3) communication with their doctors.
Methodology
Globally, 11,878 respondents made up of 9,179 patients and 2,699 caregivers took part (90+ countries) in the LC 2020 GPS. This analysis compared a subgroup of patients with lymphoma or CLL who had been in a clinical trial (n=939) ('CT patients') against a subgroup of patients who had never been in a clinical trial, but who received or were currently receiving any form of treatment for their lymphoma/CLL (n=5079) ('non-CT patients').
Demographics of both patient groups were examined, and questions relating to patients' disease management and experiences, decision-making, and patient-doctor communication were analysed. Univariate and bivariate analysis were completed as needed, and the statistical analyses were performed with IBM SPSS v27.
Results
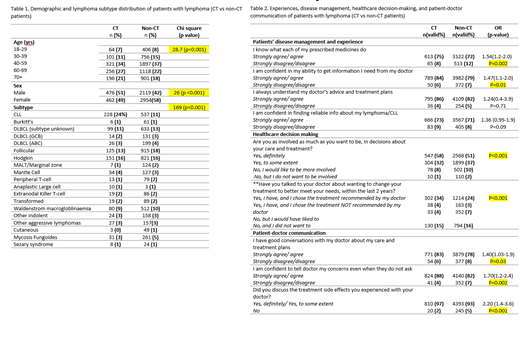
CT patients were significantly different from non-CT patients in age-grouping, sex, and subtype distribution (Table 1). CT patients had a higher proportion of older respondents (60-69 and 70+ yrs combined) compared to non-CT patients (48% vs 40%, respectively), and over half of CT patients (51%) were males compared to the non-CT group (42%).
Patients were asked to indicate how much they agree or disagree with statements relating to their experience and disease management. CT patients were 54% more likely to agree than disagree that they know what their prescribed medicines do, compared to non-CT patients [OR=1.54 (1.1-2.0); p=0.002] (Table 2). They were also 47% more likely than non-CT patients to agree than disagree that they were confident in their ability to get information from doctor [OR=1.47(1.1-2.0); p=0.01] (Table 2). Although not statistically significant, CT patients were also more prevalent in reporting that they understood their doctor's advice and treatment plans, and that they are confident in finding reliable information about their lymphoma/CLL, compared to non-CT patients (Table 2).
Patients were asked if they are involved as much as they want to be in decisions about their care and treatment (Table 2). More patients in the CT group (58%) were sure that they were as involved in decisions about their care and treatment, as much as they wanted to be, compared to non-CT patients (51%) (p<0.001). CT patients were also more prevalent (34%) in reporting that they have talked to their doctors about wanting to change their treatment to better meet their needs within the last 2 years compared to non-CT patients (24%) (p<0.001). These differences are statistically significant (Table 2).
When asked about patient-doctor communication, CT patients were 40% more likely to report having good conversations with their doctor about their care and treatment plans [OR=1.40 (1.03-1.9); p=0.03], 70% more likely to be confident in communicating their concerns to the doctor [OR=1.70 (1.2-2.4); p=0.002], and twice as likely to discuss their treatment side effects with their doctors compared to non-CT patients [OR=2.20(1.4-3.6); p<0.001] (Table 2).
Conclusion
The results show that patients with lymphoma or CLL who have been in a clinical trial generally reported being more involved in their healthcare decision-making and being more confident and having better conversations in their interaction with their doctors, compared to those patients who have never been in a clinical trial. These differences may be because patients in clinical trials have an increased accessibility to their health teams, which encourages more patient/doctor communication.
LC advocates for all patients to be informed of clinical trials they may qualify for and encourages the same level of communication between patients and doctors that occurs in a clinical trial in all patient-doctor interactions. In the future, LC would also like to explore how demographic differences may have confounded results.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal